Why wiping out HIV “reservoirs” is so hard
Virus sometimes inserts near genes that make cells divide faster.
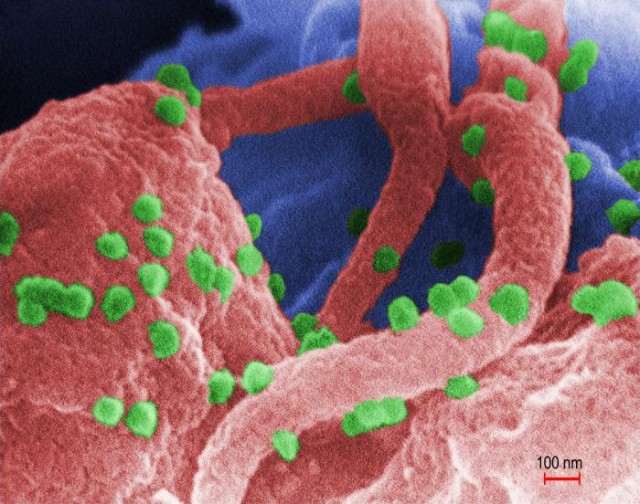
HIV (green false color) buds off an immune cell (red).
HIV, the virus that causes AIDS, does its damage by decimating immune cells. But it also lies dormant in some cells, creating a reservoir that can restart an active infection long after the active virus has been cleared by treatments. This is apparently what happened to a child from Mississippi who was thought to have been cured of infection following antiviral treatments.
Researchers may now have found one of the reasons that it's so hard to clear out these reservoirs of infected cells. As part of the infection process, HIV normally inserts a copy of itself into a cell's chromosomes. By chance, some of these insertions cause the cell to grow faster, ensuring that more copies of the virus are around to cause trouble.
The researchers, who are all based in Seattle, took a pretty simple approach to discover this: they sampled cells from HIV patients who were receiving long-term antiviral treatment, looking for the sites of HIV insertion. In these patients, viral replication was suppressed by the drugs, often for periods of over a decade. Therefore, any viruses researchers found were from those cells that had quiescent viruses inserted into their genome.
In total, the team identified over 500 individual viral insertion sites. They then figured out whether the virus was inserted in or near a gene and, if so, what that gene does. In many cases, the nearby genes were involved in controlling cell growth. Of the 20,000 or so human genes, only a bit over five percent have been associated with cancer. But over 12 percent of the genes that had HIV insertions in or near them had been implicated in cancer, and an even higher number had been found to control cell growth and proliferation.
In one patient, 31 percent of the infected cells had HIV insertions near a gene that stops cell division if DNA damage is detected. Another had seven distinct HIV insertions in a gene that acts as a tumor suppressor in immune cells (a second had two additional insertions in the same gene). In total, at least 12 genes had HIV insertions in at least two of the three patients; several of these genes have also been associated with controlling growth.
Why growth genes?
It might be tempting to speculate that HIV selectively targets those genes that are involved in growth, but the authors suggest that an evolutionary process is at play. HIV inserts at random, and some of those insertions happen to be near genes. When that's the case, the presence of the virus can alter the gene's activity, either by disrupting it or by causing it to become more active. In some cases, this will be harmful and the cell will die or grow poorly; in others, it will have little effect.
But in a few cases, the virus' insertion will affect nearby genes in a way that can cause the cell to grow faster. Either the insertion will inactivate a gene that slows down growth or it will up-regulate a gene that promotes growth. Over the course of a decade, even if the boost in growth is very small, this will cause the virus-infected cell to outgrow other infected cells, as well as uninfected cells. In short, through random chance and selection, a few insertions will ensure that there are plenty of cells with silent HIV insertions, waiting to re-establish an active infection should the chance appear.
The authors suggest that their results may be an underestimate of how often cell growth genes are involved. To begin with, some of the sites of HIV insertion may not be very close to genes yet will still be able to alter their activity. In addition, some of the genes that the authors have identified are known to affect cell growth but aren't listed that way in the major databases, so they don't show up in the list of genes associated with cancer.
The results highlight an unfortunate fact of HIV: while learning how to control the virus was hard, learning how to eradicate it is going to be really hard. Scientists have lots of ideas about how to go about this, but until one of them makes some substantial progress, any talk of an actual "cure" is premature.
Science, 2014. DOI: 10.1126/science.1256304 (About DOIs).
No comments:
Post a Comment